- Life
- Research
Published: | By: Alena Gold
Biologists and chemists at the University of Jena decipher properties of a bacterial toxin that renders the unicellular green algae Chlamydomonas reinhardtii motionless. The toxin appears to hijak the calcium channels of the target algae, causing a rapid influx of calcium ions that within one minute results in the loss of the algal flagella and therefore the algal means of escape. The researchers published their study in the current issue of the journal "New Phytologist".
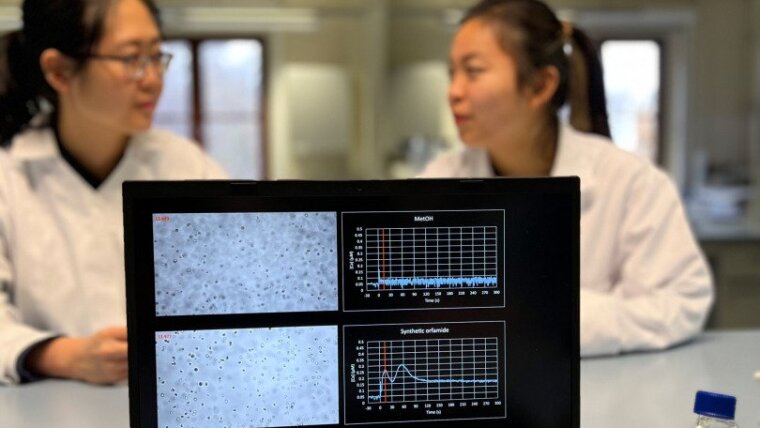
Like a predator in the microverse, the bacterium Pseudomonas protegens "hunts down" its algal victim Chlamydomonas reinhardtii using the toxin orfamide A, which can immobilize the green algae within a very short time and prevent them from swimming to the light to perform photosynthesis. The team led by Prof. Dr. of General Botany Maria Mittag studied the mode of action of the toxin, which is composed of a peptide with a long-chain fatty acid tail. Together with Prof. Dr. Hans-Dieter Arndt and doctoral student Yuko Bando, both from the Institute of Organic Chemistry and Macromolecular Chemistry, as well as other researchers, Mittag and doctoral student Yu Hou found that the toxin function is highly dependent on minor structural elements. As soon as the lipid portion is too short, is removed from the peptide, or the form of certain amino acid building blocks changes, the toxin is no longer efficacious. It specifically triggers certain calcium channels of the so-called TRP (transient receptor potential) type of the alga. But if one of these channels is switched off, for example by mutation, the proportion of calcium ions absorbed decreases and the tendency to flagellation decreases.
Team of biologists and chemists jointly decode the mode of action of the toxin
Using chemical synthesis, Prof. Arndt's team not only re-created the toxin in the laboratory, but also created variants with modified chemical building blocks. These variants were then comparatively examined by the team of Prof. Mittag for their ability to trigger an influx of calcium ions into the algal cells. Furthermore, mutants of calcium channels were used to find the original targets of the bacterial attack. At least four TRP-type calcium channels are involved. Such channels are often sensors for thermal or even chemical stimuli and may even be located directly in the flagella of the algae.
With their basic research, the researchers in the CRC "ChemBioSys"External link are looking for natural substances that mediate the interaction of microorganisms, and in the Cluster of Excellence "Balance of the Microverse"External link for the underlying mechanisms and their influence on the host, in this case the microscopic algae. Microalgae contribute greatly to the fixation of the greenhouse gas carbon dioxide, as well as to global oxygen production. Although they live closely together in nature with bacteria and fungi, which can influence their fitness positively or negatively, knowledge about these essential interactions and their influence on our environment is so far very limited.
Original publication:
Hou et al. A cyclic lipopeptide produced by an antagonistic bacterium relies on its tail and TRP-type Ca2+ channels to immobilize a green alga. New Phytologist 2022, doi: 10.1111/nph.18658. http://doi.org/10.1111/nph.18658External link